There are many variables that affect.
Marble chips and hydrochloric acid experiment method.
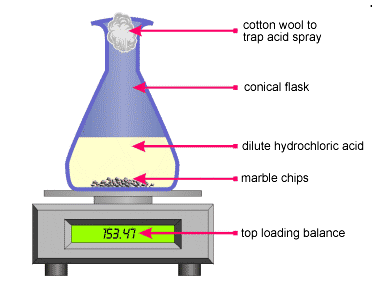
This experiment is to show how much carbon dioxide is produced during the reaction between an acid hydrochloric acid and marble.
Investigating the rate of reaction between marble chips and the varying concentrations of hydrochloric acid planning before starting the experiment i had to work out how much marble chips would be needed and how much hcl would be needed.
Hydrochloric acid to react with the marble chips independent variable marble chips to react with the acid dependent variable stopwatch to accurately time the experiment spatula to handle the marble chips measuring cylinder to precisely measure out different concentrations of hydryochloric acid electric balance to measure the mass g of the marble chips bung.
Hydrochloric acid marble chips the experiment the aim of this experiment is to find out how different variables affect the rate at which the reaction between marble chips caco and hydrochloric acid hcl takes place.
Investigating the rate of reaction between marble chips and the varying concentrations of hydrochloric acid 705 words 3 pages.
Plugged in scientific scales and weighed out 1g of marble chips for each test tube.
Marble chips placed onto pieces of paper.
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
Measured 5ml of hydrochloric acid in the 10ml measuring cylinders and placed into each beaker separately.
Measured out 1ml of water in a 10ml measuring cylinder and placed into the test tube labelled 2.